Quality & Technical Documentation
Sterixia oils are produced under a documented quality system with controlled processing, lot traceability, and batch-specific testing. The documents below are representative examples provided for technical evaluation and formulation review.
These documents reflect our standard documentation approach for cosmetic grade oils.
One-page overview of Sterixia ultra-pure cosmetic oils, processing philosophy, and intended applications.
Typical physical and chemical properties, processing overview, packaging options, and handling guidance.
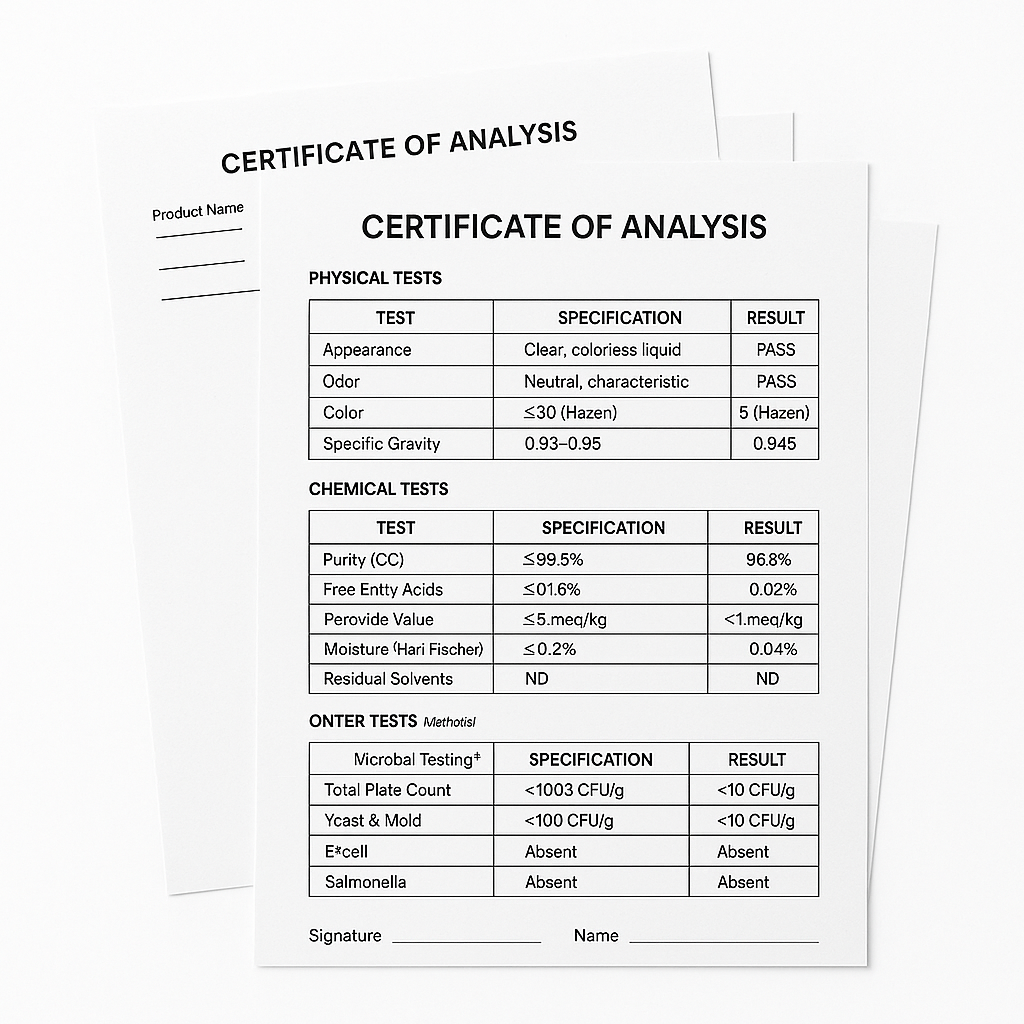
Example batch results, test methods, and quality release format for cosmetic-grade material.
What’s Included (by lot or on request)
COA provided per lot
Identity testing available (FTIR; additional methods as applicable)
Lot traceability
PV/AV reporting for oils (as applicable)
SDS and batch documentation available upon request
Why Documentation Matters for Formulators
More reproducible R&D
Fewer failed batches
Easier scale-up
Smoother internal QA reviews